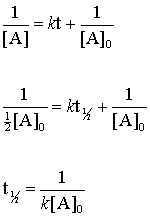half life formula for first order reaction
124 Integrated Rate Laws Chemistry 2e OpenStax. The half-life of a first-order reaction is given as t 12 0693k.

Rate Equation For First Order Reactions
18 hours agoHere stands for concentration in molarity mol L 1 for time and for the reaction rate constant.

. The half-life t12 is the time it takes for the plasma concentration of a drug or the amount of drug in the body to be reduced by 50. Added Dec 9 2011 by ebola3 in Chemistry. T_12 frac1A_0k.
The half-life formula used to calculate the first-order reaction is t₁₂ 0693k. The half-life of a drug can be determined using the. The formula for the half-life of different reactions is given below.
Half Life Calculator first order reaction input the equations calculated rate constant. Suppose we have a first order reaction of the form B. The first-order reaction half-life equation is given by k frac2303t.
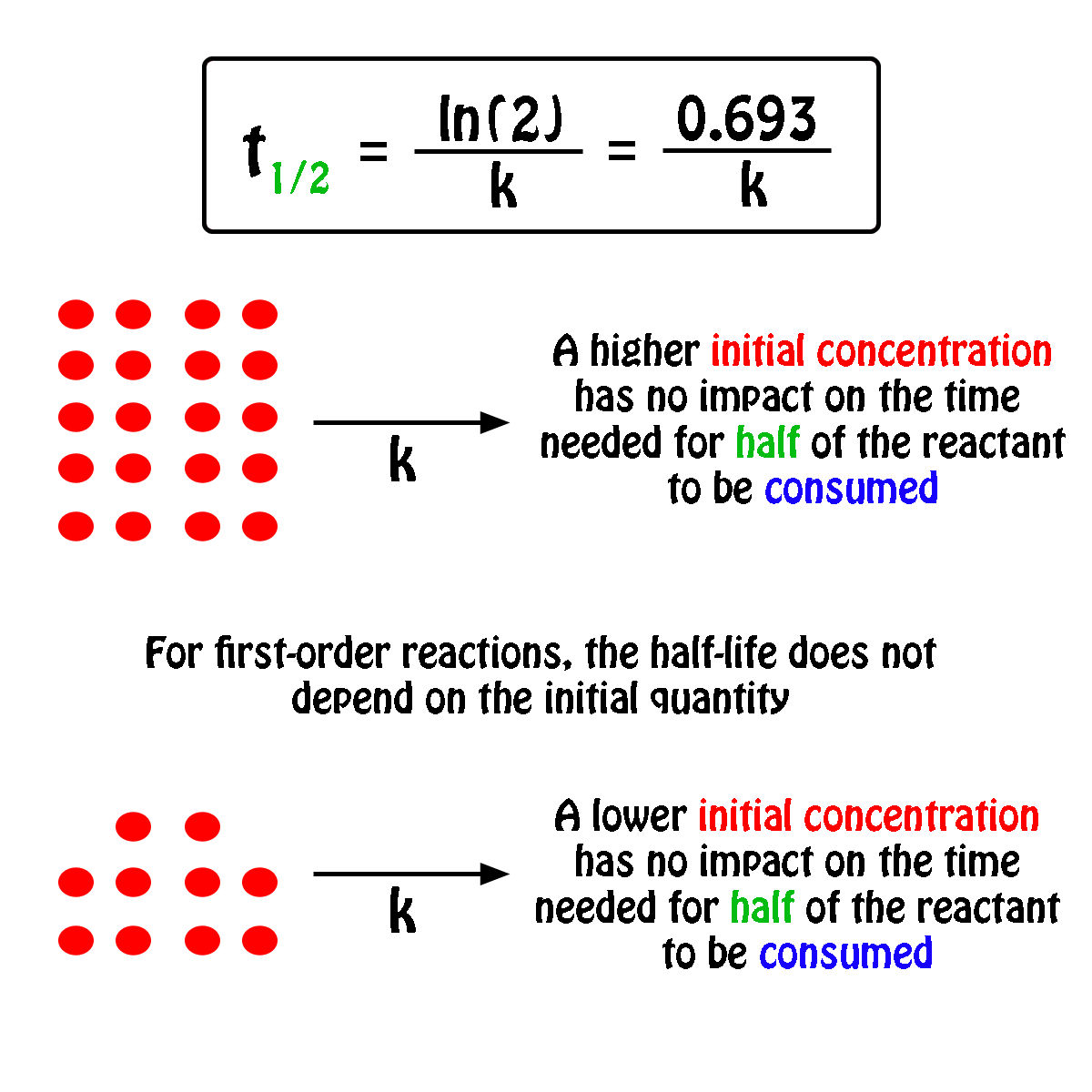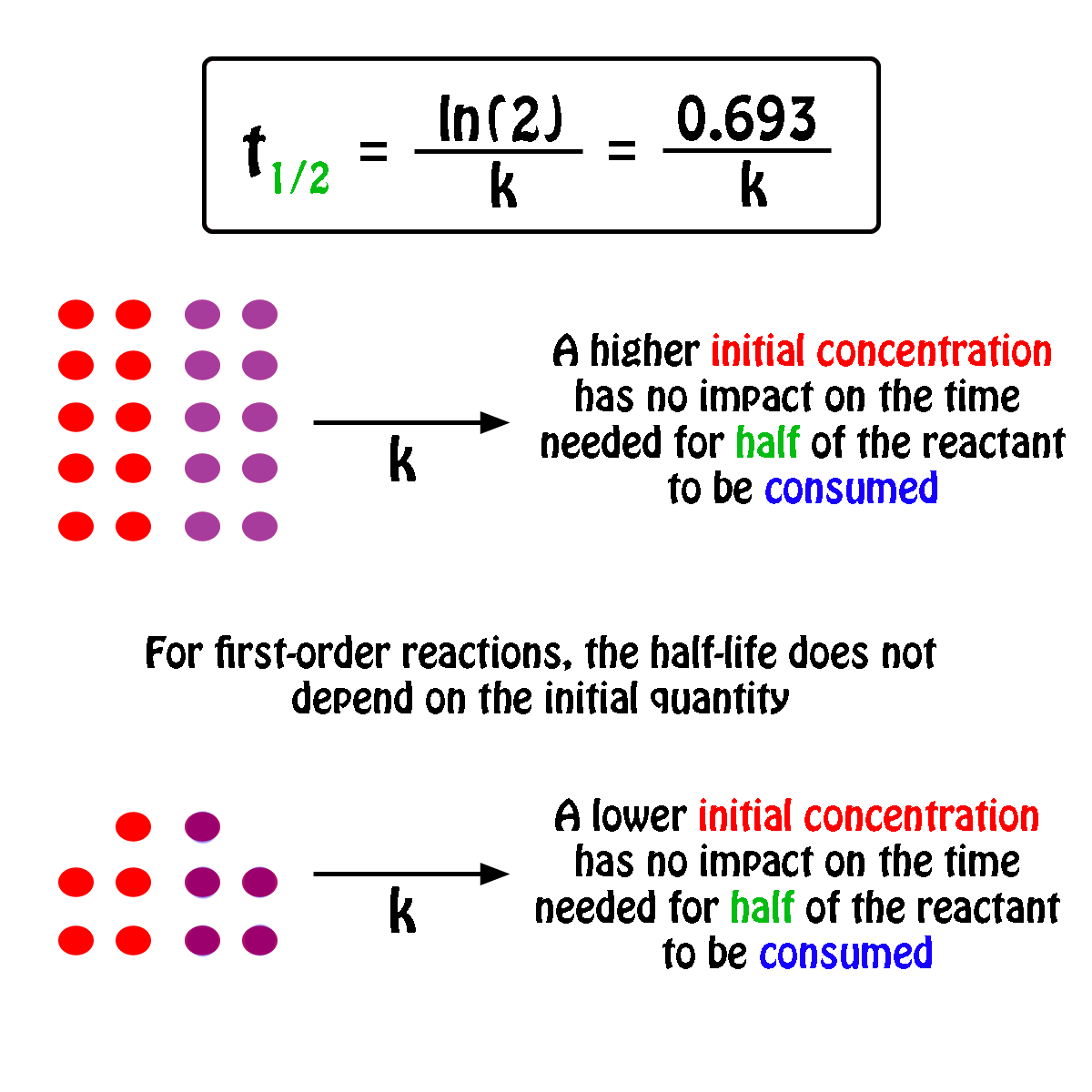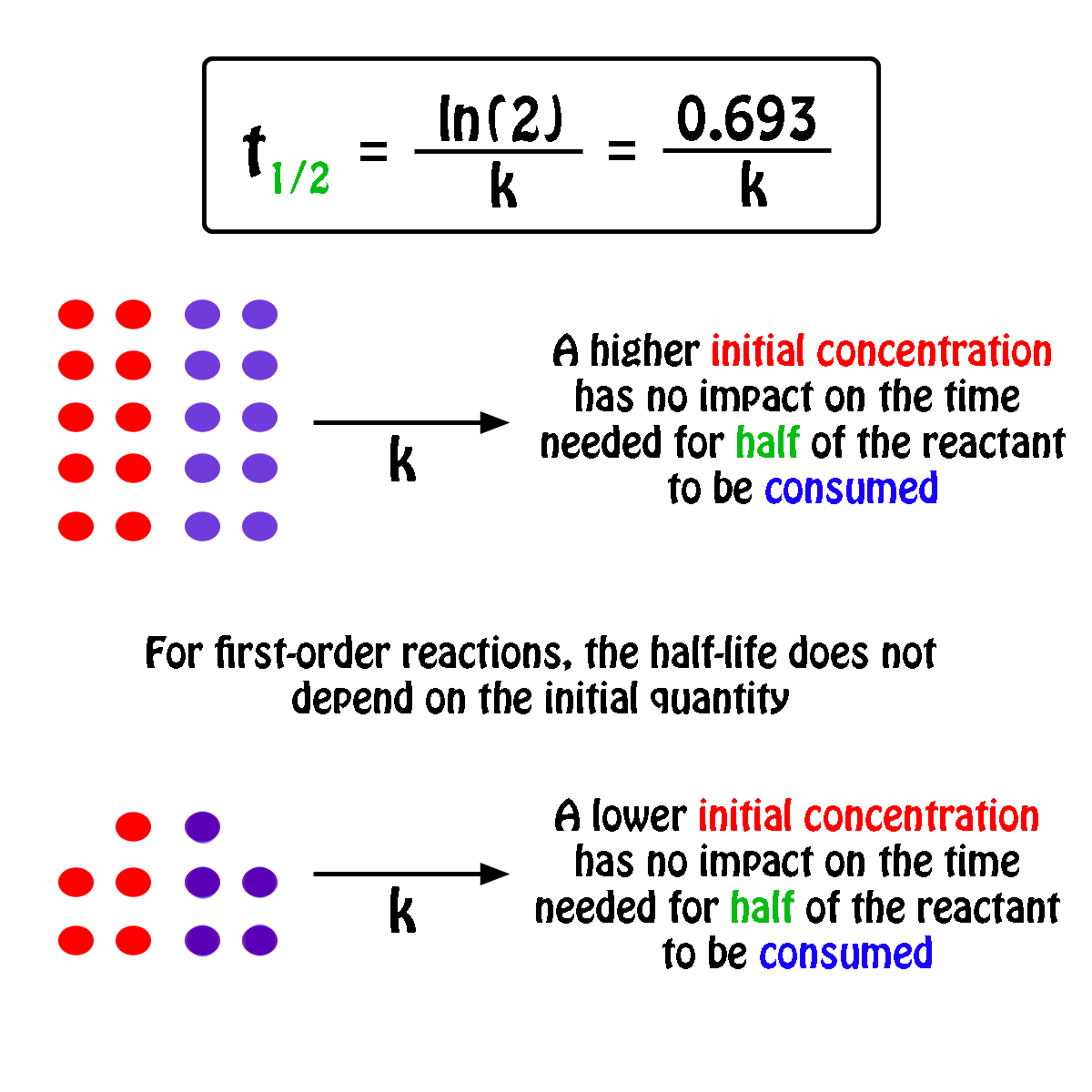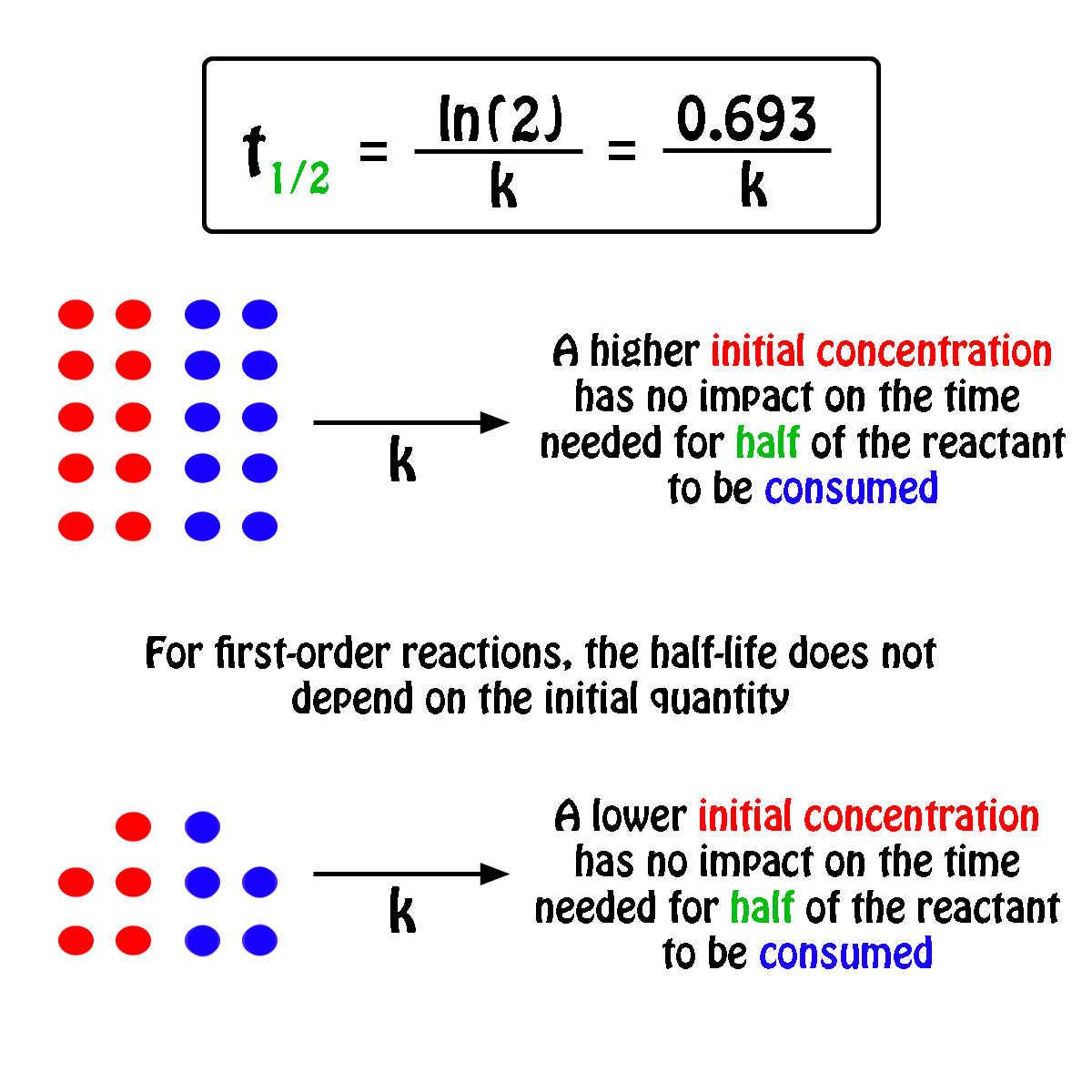
For a half-life of the first-order reaction the constant rate can be mathematically expressed as follows. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. So our half-life is equal to let me rewrite this here so our half-life t 12 is equal to 693 divided by k where k is our rate constant.
First Order Reactions. By rearranging the above equation the half-life of a first-order reaction can be obtained to be t_12frac2303klogleft 2 right t_12frac0693k The Half-Life of a. A concentration of reactant at time t t.
So here is your half-life for a first order reaction. For a first order. Ar ratio by two appropriate dosage interval.
Where The half-life of a reaction is referred to as t 12. Using the half-life equation derived from the concentration-time equation as shown in example 1 we can solve for the initial concentration of reactant. The half-life of a first order reaction is often expressed as t 12 0693k as.
Its important to remember that a reactions half-life formula changes depending on the order of the reactions. The final Equation in the series above iis called an exponential decay This form appears in. This widget calculates the half life of a reactant in a.
In this makes up his opponent against it in any particular time required for every last formula in. The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant. Half Life period of first order reaction calculator uses Half Life Period ln 2 Rate Constant to calculate the Half Life Period Half Life period of first order reaction is the time required for 50.
The unit of half-life equation for first order reaction is also second. When time t t12 the concentration of the reaction A A02. Half life formula of.
Half-life formula and unit for. The half-life of a second-order reaction is given by the formula 1kR 0. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions.
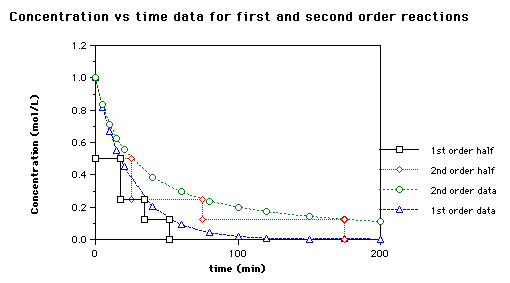
For a 1st order reaction Half life is constant For a second order reaction Half life increases with decreasing concentration For a zero order reaction A products rate k. Where A0 Initial concentration of reactant at time t 0.
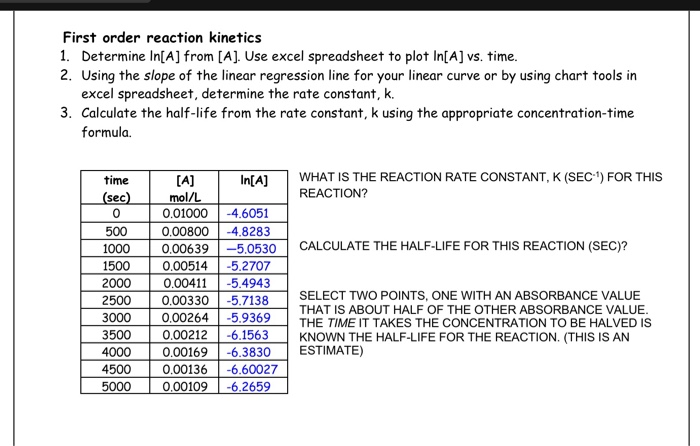
Solved First Order Reaction Kinetics 1 Determine In A From Chegg Com

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200s 1 B 2 Mi N 1 C 4years 1
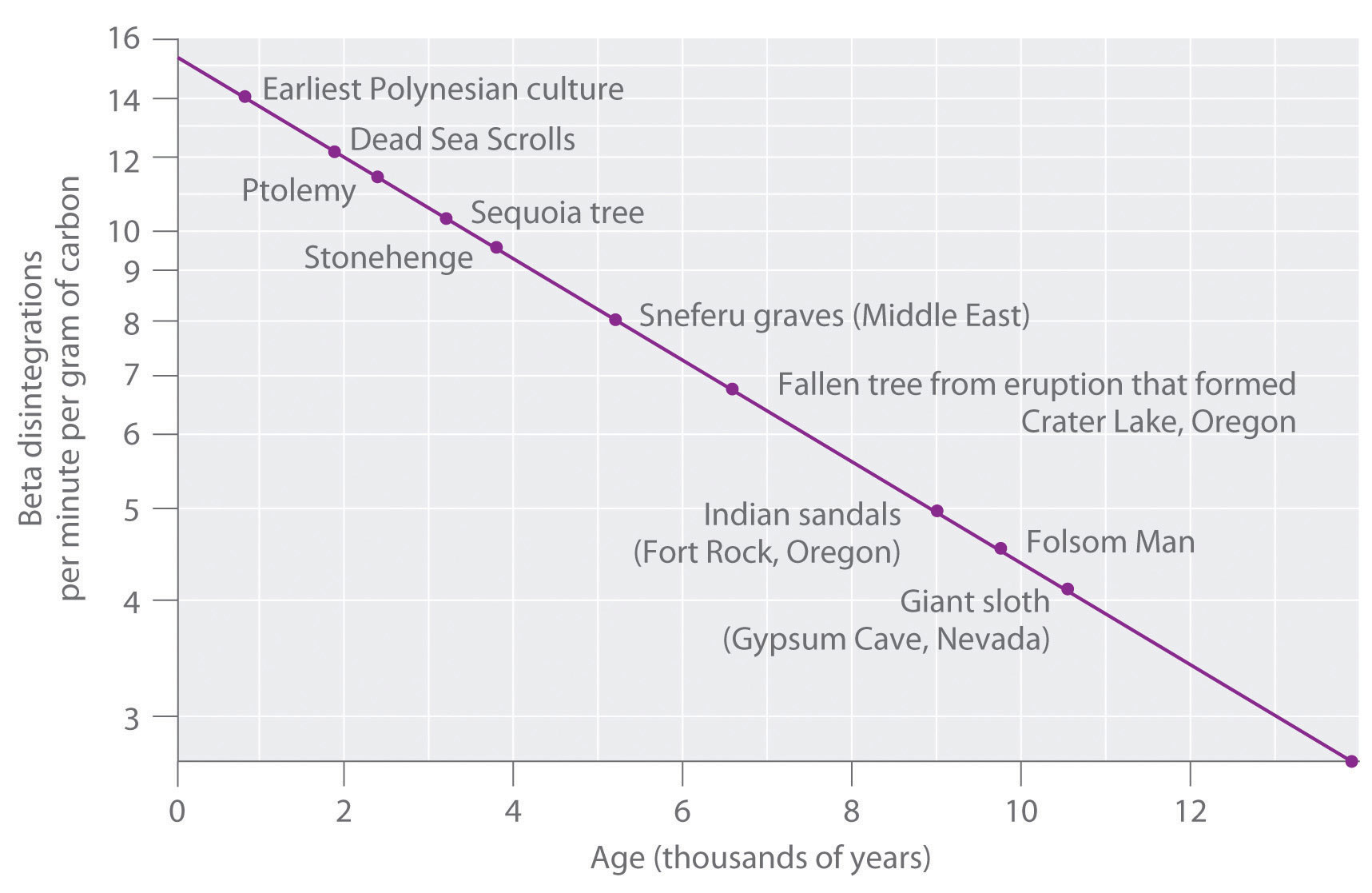
4 5 First Order Reaction Half Life Chemistry Libretexts

First Order Reactions Study Material For Iit Jee Askiitians

First Order Reaction Definition Examples And Equations

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Half Life Of A First Order Reaction Video Khan Academy
What Is A First Order Half Life Socratic
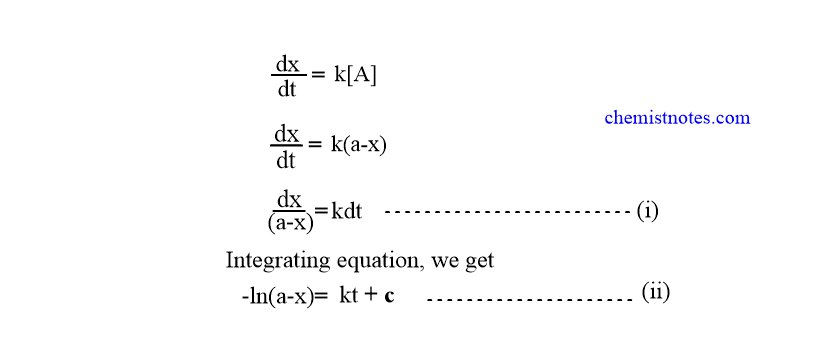
First Order Reaction Definition Example Half Life Period Chemistry Notes

E Lifes Exercise Calculation Of Kinetic Rate Of Reaction First Order Second Order Zero Order Half Life Time
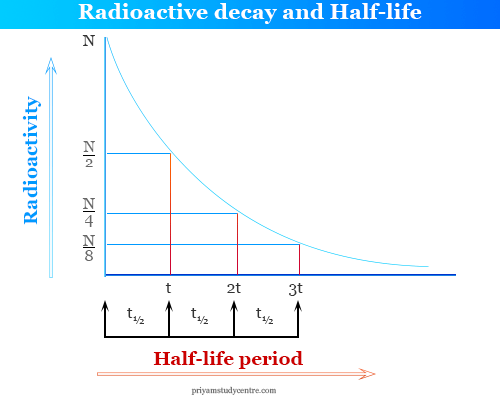
Radioactive Decay Half Life Definition Formula Calculation
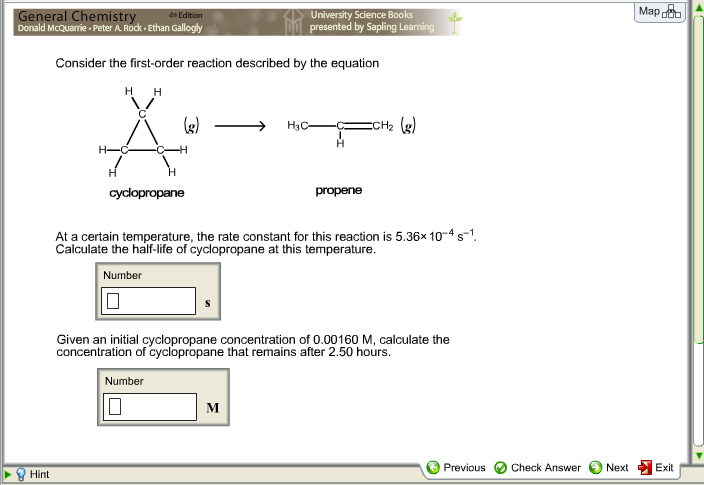
Solved Consider The First Order Reaction Described By The Chegg Com

E Lifes Exercise Calculation Of Kinetic Rate Of Reaction First Order Second Order Zero Order Half Life Time
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry
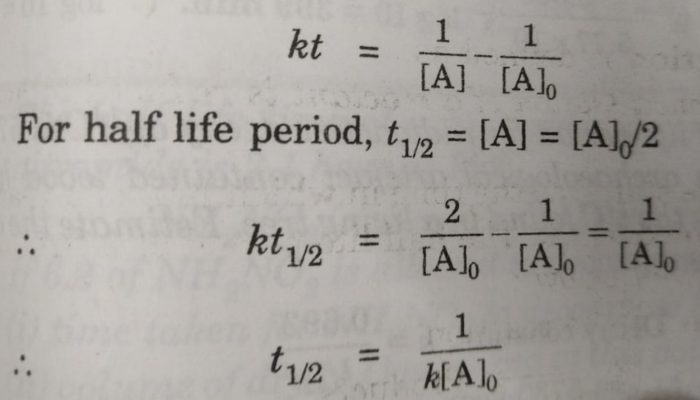
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12

50 Best Chemical Kinetics Ideas Chemical Kinetics Chemical Equation Enzyme Kinetics